Home > Medical Injection Molding
Details
Details:
Model
Material
Brand
Origin
Application
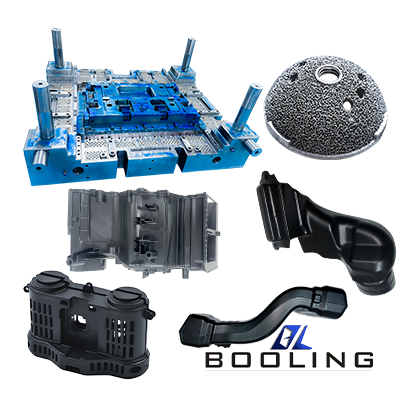
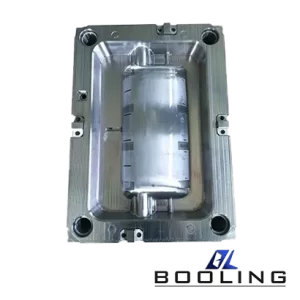
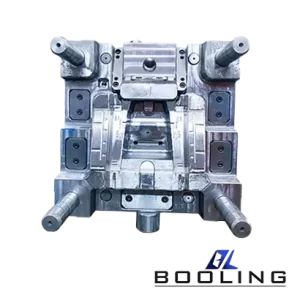
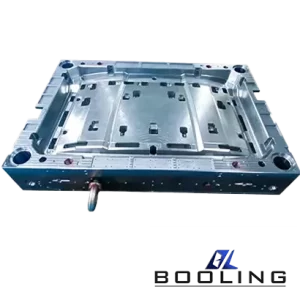
Booling-Injection Mold-1
P20, 718, S136, 2738 etc
Booling
China
Home Appliance parts
Injection molding mainly uses molds to shape various plastic products, rubber products and some metal products. Its application range is extremely wide, from automotive parts, electronic equipment casings to daily household items, medical equipment, etc., all of which are inseparable from the participation of the molding process.
Advantages of injection molding:
- High strength and toughness
- Good transparency
- Extreme temperature resistance
- Chemical corrosion resistance
- High production efficiency
Related Hot Products
In the medical field, where life and health are at stake, every tiny component carries a significant mission. As a core technology in modern medical product manufacturing, injection molding, with its advantages of high precision, high efficiency, and low cost, plays a vital role in driving the innovative development of medical devices.

Key Types of Medical Injection Molds
Medical devices are diverse, and the corresponding injection mold designs are highly specialized and targeted, requiring extremely high precision, stability, and cleanliness:
1. Disposable Consumable Molds: These molds, such as syringes, infusion connectors, urine collection containers, and reagent cups, have complex structures, require multiple cavities, and high production volumes. They also feature advanced designs such as fully automated demolding and hot runner systems to ensure an efficient and sterile production environment.
2. In Vitro Diagnostic (IVD) Consumable Molds: These molds are suitable for complex products such as microfluidic chips, test cards, sample cups, and pipette tips. These molds place extremely stringent demands on microstructure precision and dimensional stability. Mirror polishing is often used to achieve ultra-low surface resistance, ensuring smooth and residue-free fluid flow.
3. Surgical instrument component molds: These include surgical forceps parts, surgical blades, anesthesia masks, and endoscope housings. The design of these molds must consider the device’s functionality and ergonomics, prioritizing high dimensional accuracy, excellent strength stability, and superior chemical resistance. Furthermore, mold steels often utilize high-grade, corrosion-resistant materials such as S136 to ensure long-term reliability.
4. Implantable/Interventional Device Component Molds: These include orthopedic introducers and heart valve testing tools. While injection molding is rarely used for implantable devices, these components often place high demands on biocompatibility and a cleanroom environment (ISO Class 7 or higher). These components are typically produced using cleanroom injection molding in Class 10,000 or higher cleanroom facilities.
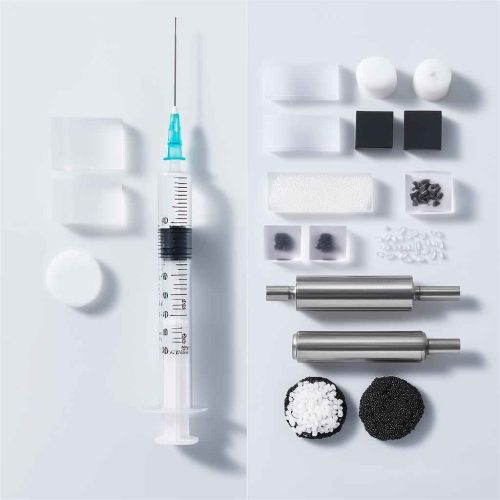
Core Requirements for Medical Device Injection Molding
The medical injection molding process extends beyond general injection molding to incorporate even more stringent “medical-grade” controls:
1. Ultra-precision Injection Molding: Key dimensions are controlled to an accuracy of ±0.001 inch (±0.025 mm), ensuring minimal repeatability and batch-to-batch consistency. Products such as IVD microfluidic chips require micron-level precision control.
2. Stack Mold Technology: This double-sided stack mold design significantly increases single-shot output while maintaining the same equipment footprint, while maintaining consistent energy consumption. This significantly reduces the cost per unit for high-volume disposable consumables such as reagent cups.
3. Hot Runner System: This eliminates cold slugs, significantly reduces runner material waste (savings can reach up to 95%), shortens molding cycles, and improves injection molding workshop cleanliness, making it particularly suitable for the production of high-volume, dust-free consumables.
4. Precise Mold Temperature Control: Mold temperature stability is controlled to within ±0.5°C to ensure consistent material crystallinity and shrinkage, which is particularly important for crystalline medical plastics (such as PP and POM).
5. Cleanroom Injection Molding: Critical medical products are manufactured entirely in ISO Class 6 or higher (Class 1000) clean environments (such as locker rooms, air showers, and sterile packaging rooms). Particle and microbial control is maintained throughout the process to ensure product sterility prior to initial packaging.
6. High-Speed Injection and Quick Mold Changes: Combining the stability of medical-grade injection molding machines, we achieve high-speed mold clamping speeds of ≥20 tons/square centimeter. Utilizing rapid mold changeover technologies (such as hydraulic cylinder-driven slides and standardized baseplates), mold changes can be completed in under 80 seconds.
7. Rigorous Validation System: Complying with ISO 13485 and FDA QSR 820 requirements, we thoroughly validate the stability of process parameters (such as pressure profiles, temperature parameters, and dwell time) to ensure batch-to-batch product consistency.
Medical-Grade Materials: Safety and Functionality
Not all plastics are suitable for medical applications. Medical-grade materials that meet biocompatibility, sterilization requirements, and long-term reliability are preferred.
1. Rigorous Certification: Requirements include USP Class VI plastic certification, ISO 10993 biocompatibility testing certification, and FDA Pharmacopoeia registration. For example, materials that comply with FDA 21 CFR and EU 10/2011 food contact standards are used.
- Precision molds: Used to produce medical device components requiring high-precision dimensions and surface finishes. Examples include microsyringes and catheter connectors.
- Multi-cavity molds: To improve production efficiency, multi-cavity molds can simultaneously produce multiple identical or different parts in a single injection molding process. These molds are ideal for mass-produced medical products, such as disposable surgical instruments.
- Hot runner molds: By using a heated runner system to maintain the temperature of the plastic melt, they reduce waste and achieve more uniform filling, making them particularly suitable for manufacturing high-quality medical components, such as certain parts for hemodialysis equipment.
- Two-color or multi-material molds: These molds allow the combination of two or more different materials or colors within the same part. This is particularly useful for manufacturing manual medical tools with soft grips or products requiring a combination of materials of varying hardness.
- Micro molds: Used to produce extremely small medical components, such as housings for micro-implants, sensors, or other small electronic devices.
2. Common Specialty Medical Materials:
- Polypropylene (PP): Highly cost-effective, widely used in syringe barrels, vials, pipettes, etc., and resistant to various sterilization methods, including autoclaving (over 20 cycles at temperatures above 121°C). Medical-grade PP shrinks approximately 1.5-2%.
- Polycarbonate (PC): Excellent transparency (light transmittance >88%) and high impact strength (unnotched impact strength >600 J/m), used in blood collection devices and surgical instrument housings. Resistant to gamma radiation and ethylene oxide sterilization, shrinkage is 0.5-0.7%.
- Polyphenylene ether (PPO/PPE): High heat resistance (HDT >200°C) and excellent dimensional stability (water absorption <0.1%) make it suitable for surgical instrument components that must withstand repeated sterilization, such as laparoscopic surgical equipment.
- Polyethylene (PE): LDPE is suitable for medicine bags and urine collection bags; UHMWPE is used in joint replacement implant components, boasting an ultra-low coefficient of friction (0.1-0.2) and excellent wear resistance.
- COC/COP: Cyclic olefin polymers offer excellent optical properties (transparency >92%), ultra-low leachability, and low protein adsorption, making them suitable for high-end diagnostic reagent containers, microfluidic chips, and gamma-ray sterilization resistance.
- PEEK & PEI: Specialty high-performance plastics, such as PEEK, offer high strength (flexural strength of 170 MPa), high-temperature resistance (continuous use up to 260°C), and X-ray transparency, making them suitable for complex implant guides and trauma devices. With a heat shrinkage as low as 0.1-0.3%, they are suitable for precision components.
- TPE/TPU: Medical thermoplastic elastomers offer excellent flexibility, tactile comfort, and sealing properties, and are commonly used in infusion pump tubing, respiratory masks, and handheld device encapsulation.
Injection molding, with its high precision, stability, and scalable production capabilities, provides powerful support for medical device manufacturing. From basic injection consumables to cutting-edge microfluidic devices, medical injection molding technology is providing increasingly powerful support for human health. Selecting the right mold process, using certified medical materials, and adhering to strict process parameters under a standard validation system are key to achieving high-quality medical injection molding products.
Exploring medical-grade injection molding solutions?
BOOLING Mold Manufacturing has 20 years of professional experience in medical mold manufacturing. With ISO 13485 certification, a Class 10,000 cleanroom injection molding facility, and multiple material biocompatibility testing reports, we provide global customers with precise, compliant, and reliable support for the mass production of medical devices. Contact us today to start your journey to high-quality medical injection molding production!
Automotive Mold Related Content
Applied’s expertise in modifying materials at atomic levels and on an ihdustrial scaleenables our customers to transform possibilities into reality.